Delivering the Cures
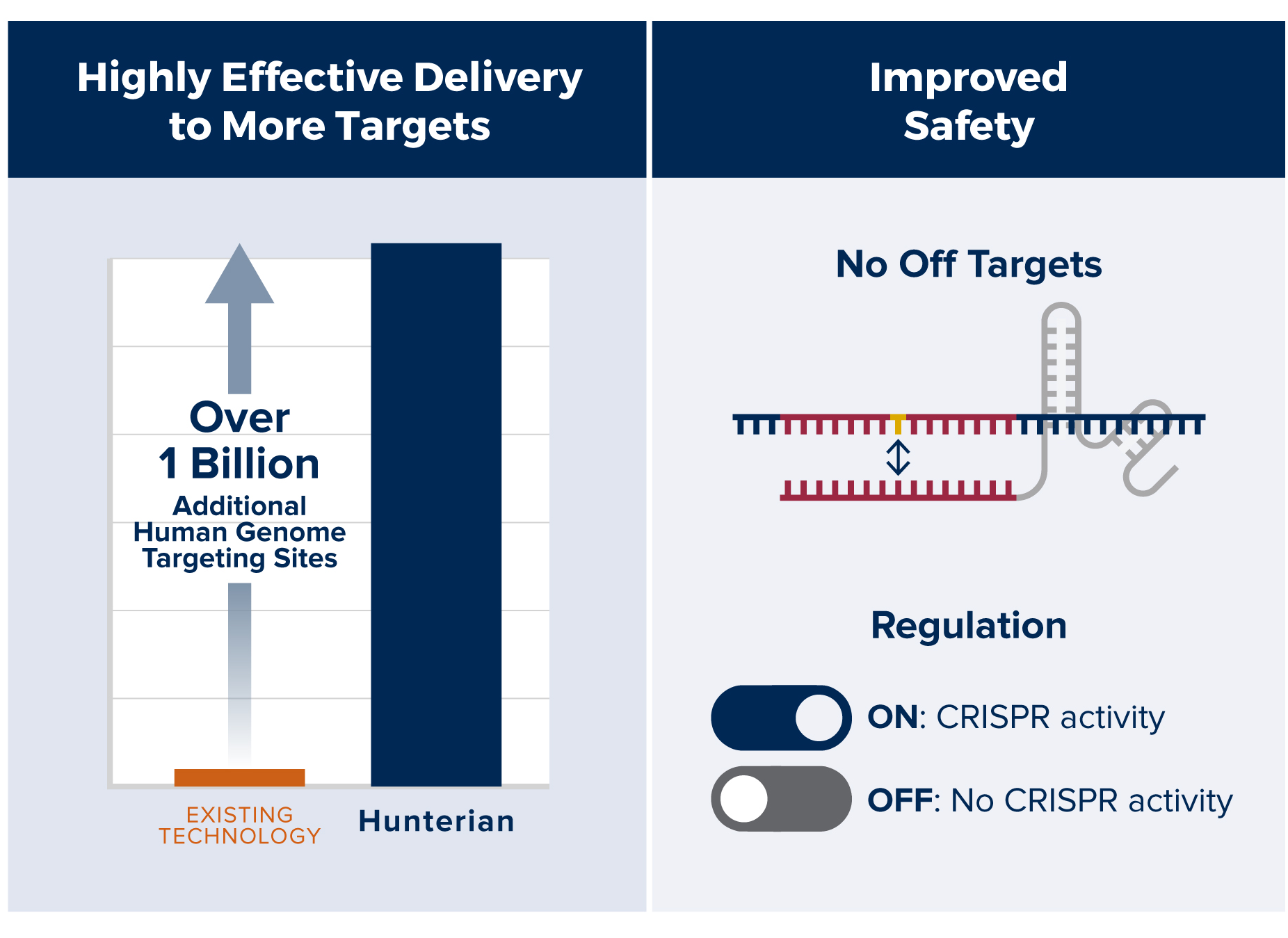
Hunterian Medicine is a gene editing company poised to unleash the full potential of CRISPR. Our patented platform technology solves the CRISPR delivery problem by enabling efficient, on-target delivery through a single adeno-associated virus (AAV). Hunterian Medicine’s technology opens an immediate path to develop cures for a vast number of genetic diseases where no FDA approved therapies exist.